VAP2B's metalloprotease-disintegrin-cystine rich (MDC) motif
MDC proteins such as VAP2B are a significant component of snake venom. The MDC motif is conserved in ADAM proteins, both by sequence and structural homology. In snake venom, an integrin binding site lies on the highlighted disintegrin loop.
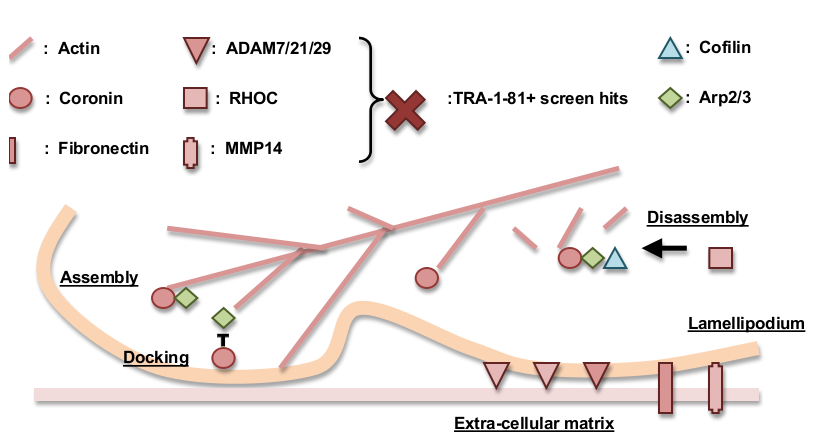
Cell adhesion and motility in reprogramming
Using a combination of gene network analysis and gene ontology analysis we identified an enrichment for cell motility and adhesion genes, among the top 5% significance level (p=0.0502) TRA-1-81+ screen hits. Cell motility and lamellipodia formation requires the controlled turnover of actin filament, which is regulated by coronin and RHOC [1-2]. Coronin positively regulates filament assembly, branching, and (in collaboration with RHOC and cofilin) filament disassembly as well; coronin inhibits membrane docking [3-5]. Coronin, RHOC, and actin are all TRA-1-81+ screen hits (CORO1A p=0.0122, CORO1B p=0.0246, ACTG1 p=0.03, RHOC p=0.0302). Other screen hits associated with cell motility and adhesion are MMP14 (p=0.0288), ADAM21 (p=0.0006), and ADAM29 (p=0.0176). MMP14 is involved in cell motility, extracellular matrix degradation, and can be involved in cancer metastasis. MMP14’s metalloprotease domain cleaves cell adhesion molecules such as cadherin and negatively regulates cell adhesion in a context specific fashion [6-7]. ADAM family proteins mediate cleavage of cell adhesion molecules E-cadherin, N-cadherin, epithelial cell adhesion molecule (EpCAM), and PTPμ subfamily receptor protein tyrosine phosphatases (RPTP) in response to ligand binding [8]. ADAMs proteins contain a metalloprotease-disintegrin-cystine rich motif similar to those found in snake venom proteins. ADAM7/21/29's disintegrin domain bears sequence homology to the blood coagulation inhibiting disintegrin domain IPR001762, which is known to regulate integrin dependent cell adhesion [9].
- Bravo-Cordero, J. J., Oser, M., Chen, X., Eddy, R., Hodgson, L., & Condeelis, J. (2011). A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Current biology : CB, 21(8), 635–44. doi:10.1016/j.cub.2011.03.039
- Cai, L., Makhov, A. M., Schafer, D. a, & Bear, J. E. (2008). Coronin 1B antagonizes cortactin and remodels Arp2/3-containing actin branches in lamellipodia. Cell, 134(5), 828–42. doi:10.1016/j.cell.2008.06.054
- Campellone, K. G., & Welch, M. D. (2010). A nucleator arms race: cellular control of actin assembly. Nature reviews. Molecular cell biology, 11(4), 237–51. doi:10.1038/nrm2867
- Chan, K. T., Creed, S. J., & Bear, J. E. (2011). Unraveling the enigma: progress towards understanding the coronin family of actin regulators. Trends in cell biology, 21(8), 481–8. doi:10.1016/j.tcb.2011.04.004
- Gandhi, M., Achard, V., Blanchoin, L., & Goode, B. L. (2009). Coronin switches roles in actin disassembly depending on the nucleotide state of actin. Molecular cell, 34(3), 364–74. doi:10.1016/j.molcel.2009.02.029
- Covington, M. D., Burghardt, R. C., & Parrish, A. R. (2006). Ischemia-induced cleavage of cadherins in NRK cells requires MT1-MMP (MMP-14). American journal of physiology. Renal physiology, 290(1), F43–51. doi:10.1152/ajprenal.00179.2005
- Polette, M., Nawrocki-Raby, B., Gilles, C., Clavel, C., & Birembaut, P. (2004). Tumour invasion and matrix metalloproteinases. Critical reviews in oncology/hematology, 49(3), 179–86. doi:10.1016/j.critrevonc.2003.10.008
- Primakoff P, Myles DG. The ADAM gene family: surface proteins with adhesion and protease activity. Trends in genetics : TIG. 2000;16(2):83-7
- Francisco S. Mini-Review ADAM, a Novel Family of Membrane Proteins Containing A Disintegrin And Metalloprotease Domain: Multipotential Functions in Cell-Cell and Cell-Matrix Interactions. October. 1995;131(2):275-278.